Pressure (symbol: p or P) is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled gage pressure)[a] is the pressure relative to the ambient pressure.
Various units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the SI unit of pressure, the pascal (Pa), for example, is one newton per square metre; similarly, the pound-force per square inch (psi) is the traditional unit of pressure in the imperial and US customary systems. Pressure may also be expressed in terms of standard atmospheric pressure; the atmosphere (atm) is equal to this pressure and the torr is defined as 1⁄760 of this. Manometric units such as the centimetre of water, millimetre of mercury, and inch of mercury are used to express pressures in terms of the height of column of a particular fluid in a manometer.
Definition
Pressure
is the amount of force acting per unit area. The symbol for pressure is p
or P. The IUPAC
recommendation for pressure is a lower-case p. However, upper-case P
is widely used. The usage of P vs p depends on the field in which
one is working, on the nearby presence of other symbols for quantities such as power and momentum, and on writing style.
Formula
Pressure
|
|
(Stress)
|
(Strain)
|
p=F/A
where:
p is the pressure,
F is the normal force,
A is the area of the surface on contact.
Pressure
is a scalar quantity. It
relates the vector surface element (a vector normal to the surface) with the
normal force acting on it. The pressure is the scalar proportionality constant
that relates the two normal vectors:
dFn = -pdA = -pndA
The
minus sign comes from the fact that the force is considered towards the surface
element, while the normal vector points outward. The equation has meaning in
that, for any surface S in contact with the fluid, the total force exerted by
the fluid on that surface is the surface integral over S of the right-hand side of the above
equation.
It is
incorrect (although rather usual) to say "the pressure is directed in such
or such direction". The pressure, as a scalar, has no direction. The force
given by the previous relationship to the quantity has a direction, but the
pressure does not. If we change the orientation of the surface element, the
direction of the normal force changes accordingly, but the pressure remains the
same.
Pressure
is transmitted to solid boundaries or across arbitrary sections of fluid normal
to these boundaries or sections at every point. It is a fundamental
parameter in thermodynamics, and it is conjugate
to volume.
Units
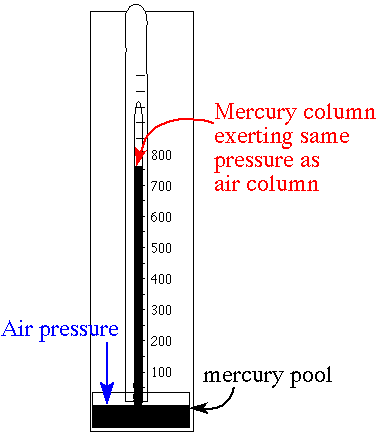

Mercury
column
The SI unit
for pressure is the pascal (Pa), equal to one newton per square metre (N/m2 or kg·m−1·s−2).
This name for the unit was added in 1971. Before
that, pressure in SI was expressed simply in newtons per square metre.
Other
units of pressure, such as pounds per square inch
and bar, are also in common use. The CGS
unit of pressure is the barye (Ba), equal to 1 dyn·cm−2
or 0.1 Pa. Pressure is sometimes expressed in grams-force or
kilograms-force per square centimetre (g/cm2 or kg/cm2)
and the like without properly identifying the force units. But using the names
kilogram, gram, kilogram-force, or gram-force (or their symbols) as units of
force is expressly forbidden in SI. The technical atmosphere
(symbol: at) is 1 kgf/cm2 (98.0665 kPa or
14.223 psi).
Since
a system under pressure has potential to perform work on its surroundings,
pressure is a measure of potential energy stored per unit volume. It is
therefore related to energy density and may be expressed in units such as joules
per cubic metre (J/m3, which is equal to Pa).
Some meteorologists prefer the hectopascal (hPa) for atmospheric
air pressure, which is equivalent to the older unit millibar (mbar). Similar pressures are given in kilopascals
(kPa) in most other fields, where the hecto- prefix is rarely used. The inch of
mercury is still used in the United States. Oceanographers usually measure
underwater pressure in decibars (dbar) because pressure in the
ocean increases by approximately one decibar per metre depth.
The standard atmosphere (atm)
is an established constant. It is approximately equal to typical air pressure
at earth mean sea level and is defined as 101325 Pa.
Because
pressure is commonly measured by its ability to displace a column of liquid in
a manometer, pressures are often expressed as a depth of a
particular fluid (e.g., centimetres of water, millimetres of mercury or inches of mercury). The most common choices are mercury (Hg) and water;
water is nontoxic and readily available, while mercury's high density allows a shorter
column (and so a smaller manometer) to be used to measure a given pressure. The
pressure exerted by a column of liquid of height h and density ρ
is given by the hydrostatic pressure equation p = ρgh, where g
is the gravitational acceleration.
Fluid density and local gravity can vary from one reading to another depending
on local factors, so the height of a fluid column does not define pressure
precisely. When millimetres of mercury or inches of mercury are quoted today,
these units are not based on a physical column of mercury; rather, they have
been given precise definitions that can be expressed in terms of SI units. One
millimetre of mercury is approximately equal to one torr.
The water-based units still depend on the density of water, a measured, rather
than defined, quantity. These manometric units are still encountered in
many fields. Blood pressure is measured
in millimetres of mercury in most of the world, and lung pressures in
centimetres of water are still common.
Underwater divers use the metre sea water (msw or MSW) and
foot sea water (fsw or FSW) units of pressure, and these are the standard units
for pressure gauges used to measure pressure exposure in diving chambers and personal decompression computers. A msw is defined as 0.1 bar,
and is not the same as a linear metre of depth, and 33.066 fsw =
1 atm. Note that the pressure conversion from msw to fsw is different from
the length conversion: 10 msw = 32.6336 fsw, while 10 m =
32.8083 ft
Gauge
pressure is often given in units with 'g' appended, e.g. 'kPag', 'barg' or
'psig', and units for measurements of absolute pressure are sometimes given a
suffix of 'a', to avoid confusion, for example 'kPaa', 'psia'. However, the US National
Institute of Standards and Technology recommends that, to avoid
confusion, any modifiers be instead applied to the quantity being measured
rather than the unit of measure. For
example, "pg = 100 psi" rather than "p
= 100 psig".
Differential
pressure is expressed in units with 'd' appended; this type of measurement is
useful when considering sealing performance or whether a valve will open or
close.
Presently
or formerly popular pressure units include the following:
- atmosphere (atm)
- manometric units:
- centimetre, inch, and millimetre of mercury (torr)
- Height of
equivalent column of water, including millimetre (mm
H
2O), centimetre (cm H
2O), metre, inch, and foot of water - imperial and customary units:
- kip, short ton-force, long ton-force, pound-force, ounce-force, and poundal per square inch
- short ton-force and long ton-force per square inch
- fsw (feet sea water) used in underwater diving, particularly in connection with diving pressure exposure and decompression
- non-SI metric units:
- bar, decibar, millibar
- msw (metres sea water), used in underwater diving, particularly in connection with diving pressure exposure and decompression
- kilogram-force, or kilopond, per square centimetre (technical atmosphere)
- gram-force and tonne-force (metric ton-force) per square centimetre
- barye (dyne per square centimetre)
- kilogram-force and tonne-force per square metre
- sthene per square metre (pieze)
 Examples
Examples
The effects
of an external pressure of 700bar on an aluminum cylinder with 5mm wall
thickness
As an
example of varying pressures, a finger can be pressed against a wall without
making any lasting impression; however, the same finger pushing a thumbtack can easily damage the wall. Although the force applied to the surface is the same, the thumbtack applies more pressure because the point concentrates that force into a smaller area. Pressure is transmitted to solid boundaries or across arbitrary sections of fluid normal to these boundaries or sections at every point. Unlike stress, pressure is defined as a scalar quantity. The negative gradient of pressure is called the force density.
Another example is of a common knife. If we try to cut a fruit with the flat side it obviously will not cut. But if we take the thin side, it will cut smoothly. The reason is that the flat side has a greater surface area (less pressure) and so it does not cut the fruit. When we take the thin side, the surface area is reduced and so it cuts the fruit easily and quickly. This is one example of a practical application of pressure.
For gases, pressure is sometimes measured not as an absolute pressure, but relative to atmospheric pressure; such measurements are called gauge pressure. An example of this is the air pressure in an automobile tire, which might be said to be "220 kPa (32 psi)", but is actually 220 kPa (32 psi) above atmospheric pressure. Since atmospheric pressure at sea level is about 100 kPa (14.7 psi), the absolute pressure in the tire is therefore about 320 kPa (46.7 psi). In technical work, this is written "a gauge pressure of 220 kPa (32 psi)". Where space is limited, such as on pressure gauges, name plates, graph labels, and table headings, the use of a modifier in parentheses, such as "kPa (gauge)" or "kPa (absolute)", is permitted.
In non-SI technical work, a gauge pressure of 32 psi is sometimes written as "32 psig" and an absolute pressure as "32 psia", though the other methods explained above that avoid attaching characters to the unit of pressure are preferred.[6]
Gauge pressure is the relevant measure of pressure wherever one is interested in the stress on storage vessels and the plumbing components of fluidics systems. However, whenever equation-of-state properties, such as densities or changes in densities, must be calculated, pressures must be expressed in terms of their absolute values. For instance, if the atmospheric pressure is 100 kPa, a gas (such as helium) at 200 kPa (gauge) (300 kPa [absolute]) is 50% denser than the same gas at 100 kPa (gauge) (200 kPa [absolute]). Focusing on gauge values, one might erroneously conclude the first sample had twice the density of the second one.
Scalar nature
In a static gas, the gas as a whole does not appear to move. The individual molecules of the gas, however, are in constant random motion. Because we are dealing with an extremely large number of molecules and because the motion of the individual molecules is random in every direction, we do not detect any motion. If we enclose the gas within a container, we detect a pressure in the gas from the molecules colliding with the walls of our container. We can put the walls of our container anywhere inside the gas, and the force per unit area (the pressure) is the same. We can shrink the size of our "container" down to a very small point (becoming less true as we approach the atomic scale), and the pressure will still have a single value at that point. Therefore, pressure is a scalar quantity, not a vector quantity. It has magnitude but no direction sense associated with it. Pressure acts in all directions at a point inside a gas. At the surface of a gas, the pressure force acts perpendicular (at right angle) to the surface.A closely related quantity is the stress tensor σ, which relates the vector force
This tensor may be expressed as the sum of the viscous stress tensor minus the hydrostatic pressure. The negative of the stress tensor is sometimes called the pressure tensor, but in the following, the term "pressure" will refer only to the scalar pressure.
According to the theory of general relativity, pressure increases the strength of a gravitational field (see stress–energy tensor) and so adds to the mass-energy cause of gravity. This effect is unnoticeable at everyday pressures but is significant in neutron stars, although it has not been experimentally tested.
Pressure Units Tabel
PRESSURE UNITS
|
|||||||
(Pa)
|
(bar)
|
(at)
|
(atm)
|
(Torr)
|
(psi)
|
||
1 Pa
|
≡ 1 N/m2
|
10−5
|
1.0197×10−5
|
9.8692×10−6
|
7.5006×10−3
|
1.450377×10−4
|
|
1 bar
|
105
|
≡ 100 kPa
≡ 106 dyn/cm2
|
1.0197
|
0.98692
|
750.06
|
14.50377
|
|
1 at
|
9.80665×104
|
0.980665
|
≡ 1 kp/cm2
|
0.9678411
|
735.5592
|
14.22334
|
|
1 atm
|
1.01325×105
|
1.01325
|
1.0332
|
1
|
≡ 760
|
14.69595
|
|
1 Torr
|
133.3224
|
1.333224×10−3
|
1.359551×10−3
|
1.315789×10−3
|
≡ 1/760 atm
≈ 1 mmHg
|
1.933678×10−2
|
|
1 psi
|
6.8948×103
|
6.8948×10−2
|
7.03069×10−2
|
6.8046×10−2
|
51.71493
|
≡ 1 lbF /in2
|
|
Example reading: 1 Pa = 1 N/m2 = 10−5 bar = 1.0197×10−5 at
= 9.8692×10−6 atm = 7.5006×10−3 Torr = 1.450377×10−4 psi
